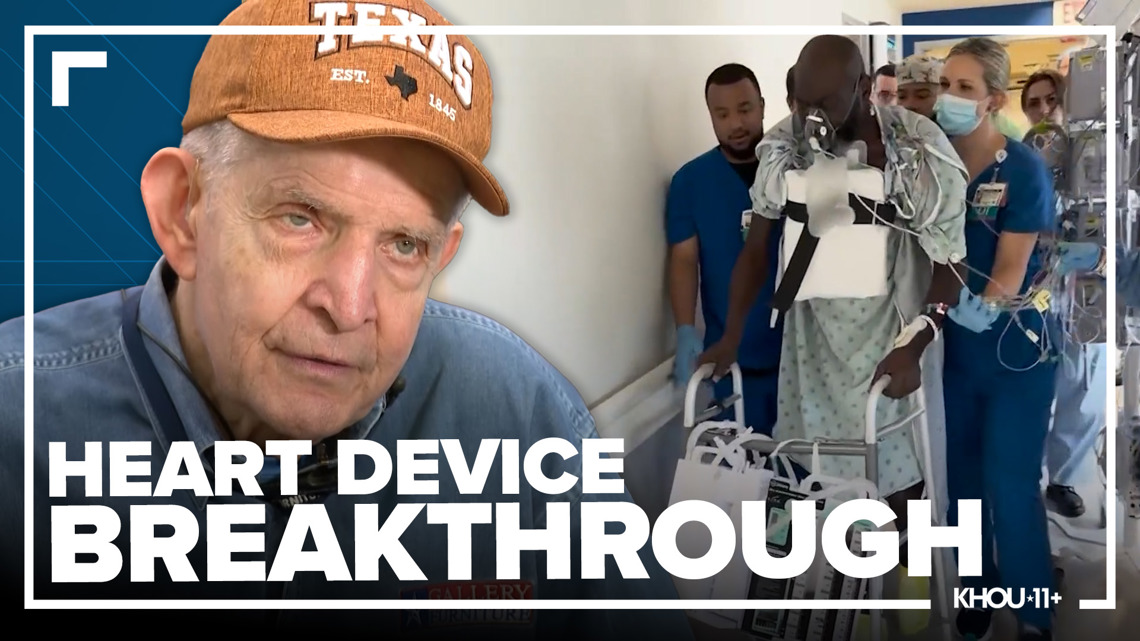
The revolutionary BiVACOR heart device was moved to Phase 2 of FDA trials after success in five human patients.
HOUSTON — In a groundbreaking move, the FDA has approved the second phase of clinical trials for the BiVACOR mechanical heart, a device that could potentially make human heart transplants a thing of the past. Fifteen additional patients will be permitted to use the experimental device and allowed to wait at home for a donor heart. The first batch of five trial patients had to be monitored in the hospital.
Like all mechanical hearts, BiVACOR currently acts as bridge for patients waiting for a human heart transplant, but its revolutionary design may make it a long term, even a permanent solution.
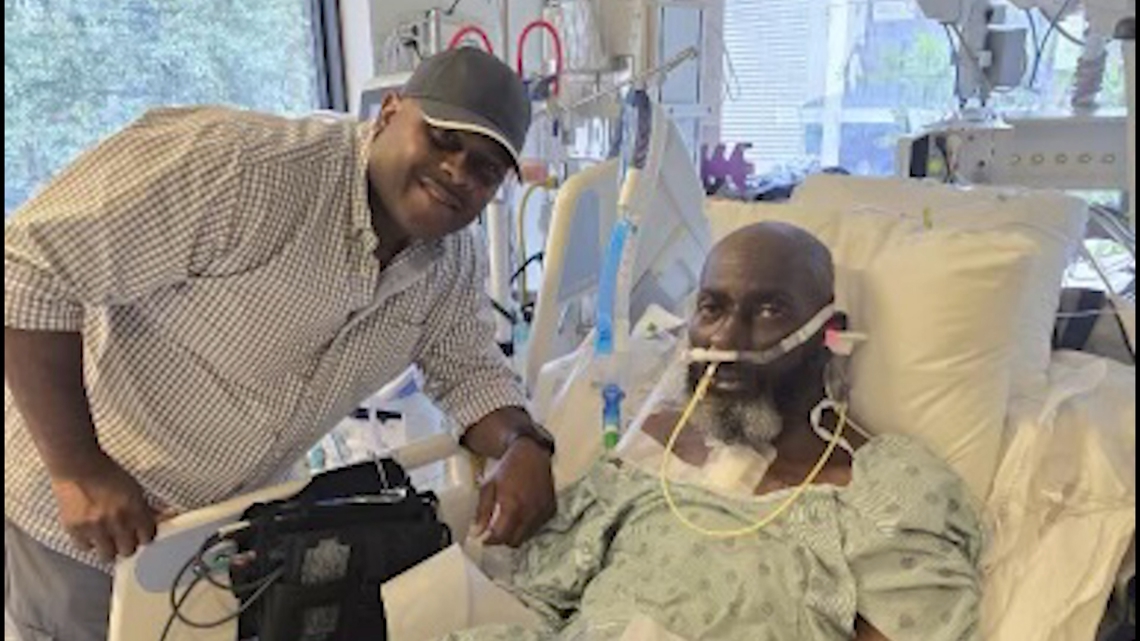
In July 2024 Batson Avie, a Hockley truck driver, became the first person ever to receive the BiVACOR device. His surgery was done at the Texas Heart Institute.
The story behind the BiVACOR heart
Approximately two and a half years ago, a normally lean Batson started experiencing severe weight gain and difficulty breathing, symptoms of congestive heart failure. He was unable to even put on his steel-toed boots, signaling that something was terribly wrong. He had congestive heart failure and needed a new heart.
Batson became so sick he couldn’t walk five or six steps. He needed a mechanical heart until a human heart became available. His condition made him a prime candidate for the BiVACOR mechanical heart, which had only been tested on animals like cows and horses. After some thought, the Avies agreed.
“We just asked God to, like, touch the doctor’s hand,” Batson’s wife Ruby said.

Batson says after surgery he felt nearly immediate relief and could breathe better walking down the hospital corridor even for the first time.
Texas Heart Institute President and C.E.O. Dr. Joseph Rogers is the principal investigator in the BiVACOR human trials. He explains, unlike traditional mechanical hearts, the BiVACOR has no valves.
“There’s only one moving part, and it’s a spinning disc inside the pump that balances the circulation between the right side of the heart and the left side of the heart,” Dr. Rogers said. “That spinning disc is suspended in a strong electromagnet.”
Nothing touches, so theoretically its parts don’t wear out. In that respect it functions similarly to a maglev train, which floats above its track. The design’s simplicity is its strength — with just one moving part versus the current mechanical heart.
It could replace the need for human heart transplants as a long term versus a temporary solution for end stage heart patients. It might be similar to artificial knees and hips.
Mattress Mack’s role in funding
The development of the BiVACOR device wouldn’t have been possible without early investors like Jim “Mattress Mack” McIngvale. The Houston-based businessman, known for his mattress sales and high-stakes gambling, took a significant risk by investing millions in Bivacor during its early stages.
“We were the first investors in BiVACOR . There would be no BiVACOR if it wasn’t for us,” said Mack. “It was a million to one shot. But I’ve been known to gamble.”
Mack’s personal connection to heart disease made his investment even more meaningful. Just months after the BiVACOR trials began, he himself underwent open heart surgery to repair a mitral valve. Three of his siblings had also undergone similar surgeries. His brother George died of congestive heart failure which runs in his family.
A new era of heart treatment
Just eight days after Batson received the BiVACOR, a human heart unexpectedly became available. He got the heart of a 34-year-old in mid-July. Now some five months after the BiVACOR and then the human heart surgeries, he feels better than he has in years, walking a mile plus daily and has lost significant water weight.
The Texas Heart Institute estimates approximately 250,000 people in the U.S. have advanced heart failure. In 2024, only about 4,200 received a heart transplant.
The BiVACOR heart could help address this crisis by replacing human heart transplants – perhaps in a decade. More engineering is needed including how to power the device more conveniently. Scientists want to create a battery system that can be implanted under the skin and recharged wirelessly, without causing burns to the skin.
To learn more about the Bivacor heart and its FDA trials, visit the Texas Heart Institute and Bivacor.
link